IR-Spectroelectrochemistry combines IR spectroscopic measurements with electrochemical investigations.
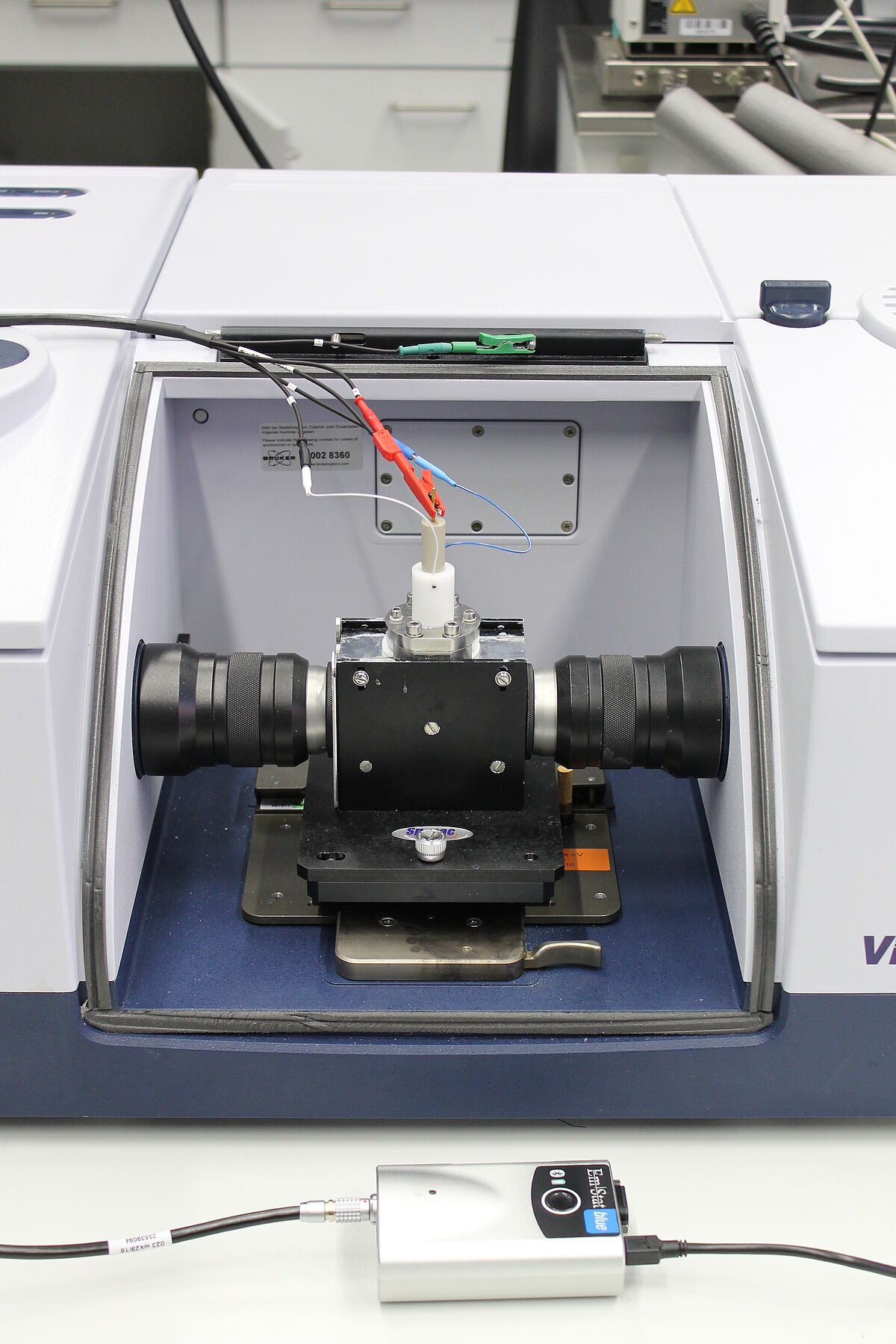
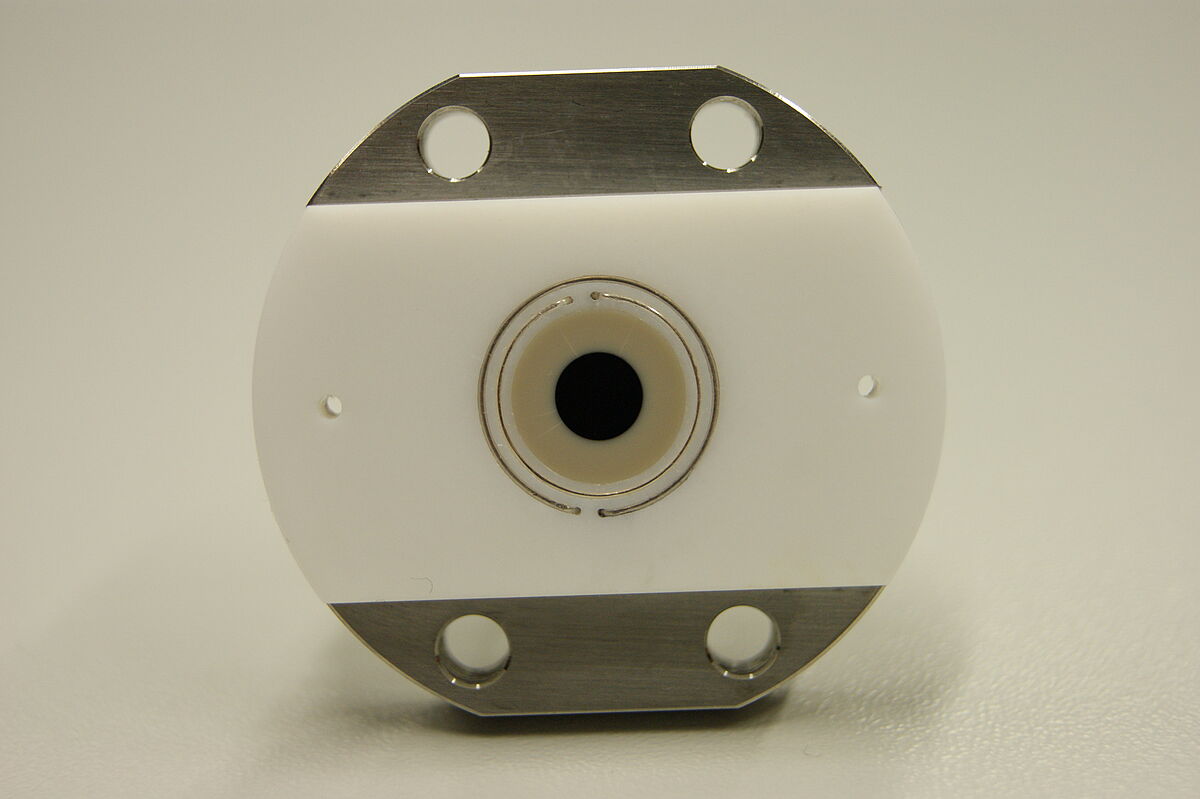
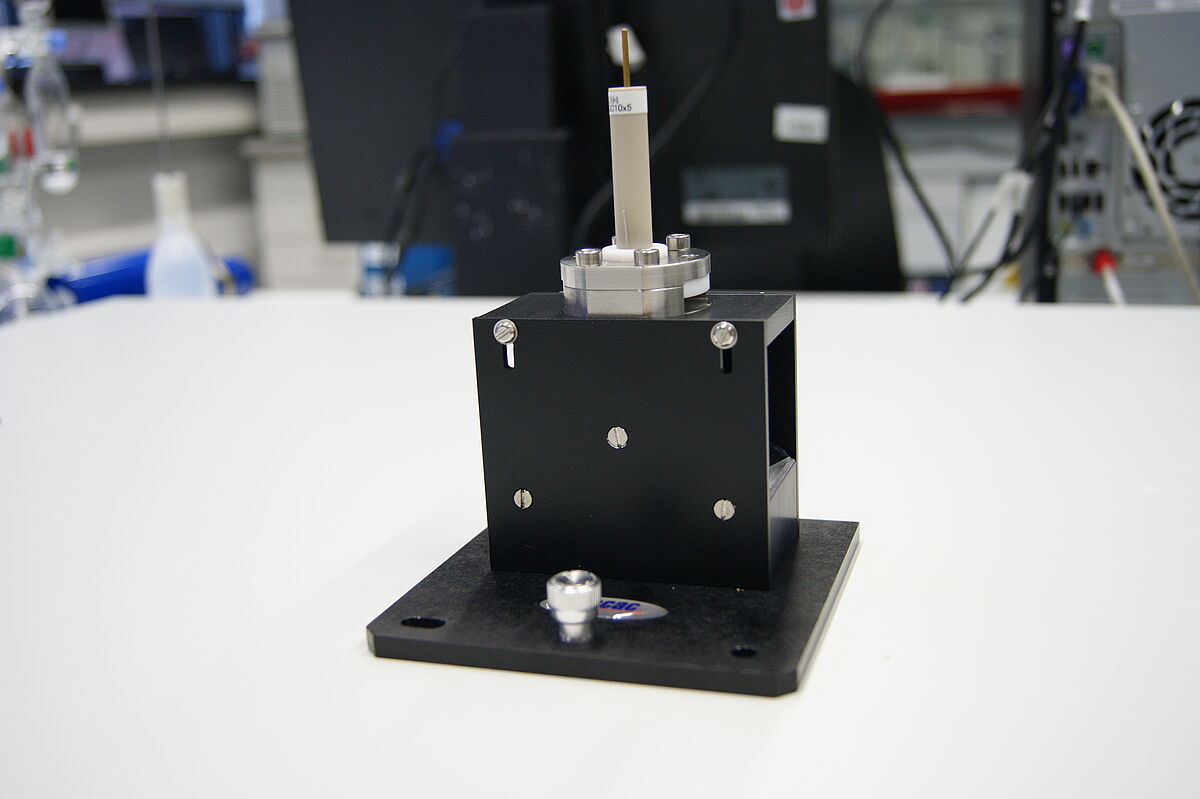
For our studies we built a spectroelectrochemical cell (SEC cell), designed by Kubiak et al., which is used in external reflection mode. Here, a polished, disc-shaped working electrode made of platinum or glassy carbon undertakes the function as IR beam reflecting surface. The pseudo reference- and counter electrode are installed in form of wires made of silver and platinum, which are circularly arranged around the working electrode. A liquid sample is located in a small layer between the electrode surface and an IR-transparent window made of CaF2.
The described spectroelectrochemical cell is mounted onto a Specac mirror reflection unit. The entire divice is coupled to a PalmSens potentiostat and installed into the BRUKER Vertex 80 spectrometer. The way of the IR beam is the following: Coming from the interferometer, the IR beam is reflected at the first mirror of the unit and passes through the CaF2 window and the sample layer. It is further reflected at the working electrode. After passing the sample layer and CaF2 window again, the IR beam is reflected for the last time at the second mirror of the unit and encounters the MCT-detector.
By application of rapid-scan-technology, the time resolution of the IR spectrometer lies within the millisecond range. In this manner, during typical electrochemical procedures like cyclovoltammetry, chemical changes of the sample as a function of the applied potential are detected by time resolved IR spectroscopy.
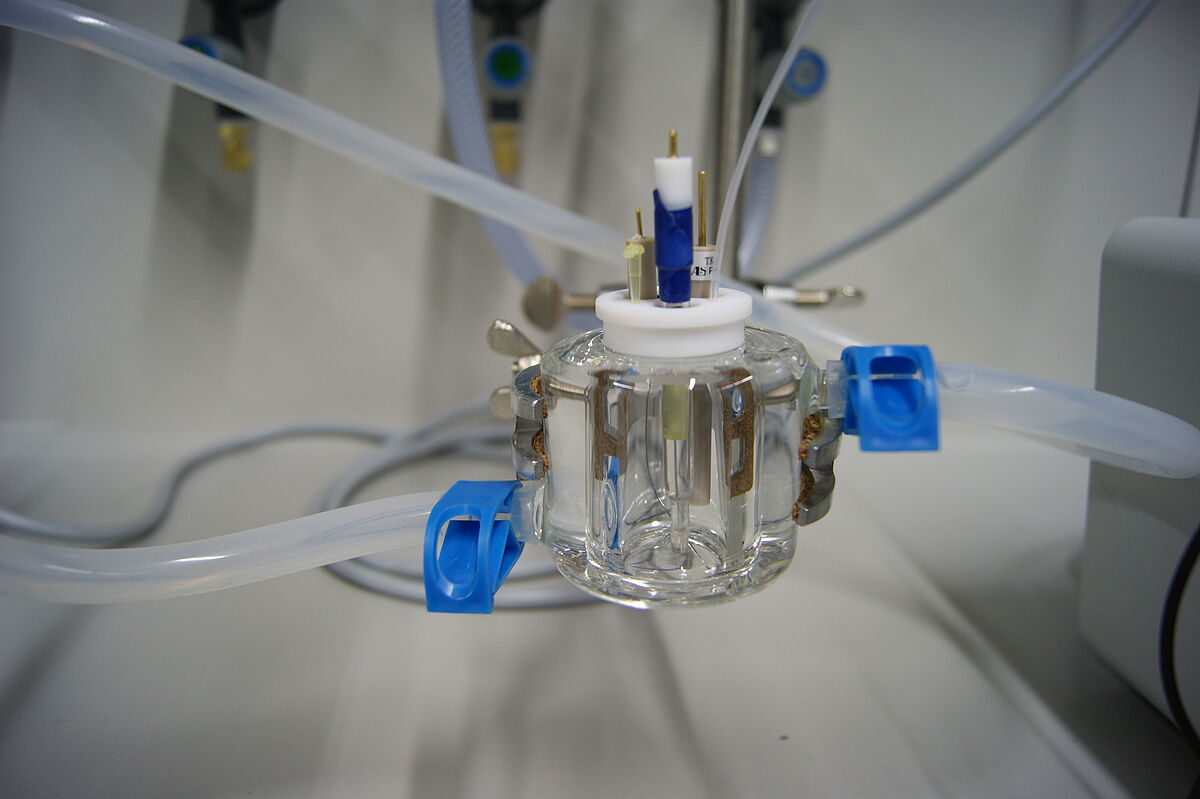
For preliminary studies we use an additional bulk electrochemistry setup with a working- and counterelectrode made of platinum, respectively, as well as a silver/silver nitrate reference electrode.